Abstract
Background
Achievement of deep molecular response (i.e., MR4.5, BCR-ABL1 ≤0.0032% on international scale, or 4.5-log reduction) is an important goal of tyrosine kinase inhibitor (TKI) treatment for patients (pts) with chronic myeloid leukemia (CML). Pts achieving a sustained MR4.5 are potential candidates for treatment-free remission. However, data on MR4.5 after TKI therapy outside of clinical trial setting are limited, especially in second-line TKI treatment setting.
Objective
This study aims to describe pt and treatment characteristics by regions and investigate frequency and time to achieving MR4.5 in pts with chronic phase (CP)-CML treated with second-line nilotinib or dasatinib in 10 countries, categorized into two regions, Europe [France, Germany, Italy, Netherlands]/Australia and Latin America - LATAM [Argentina, Brazil, Chile, Colombia, and Mexico]).
Methods
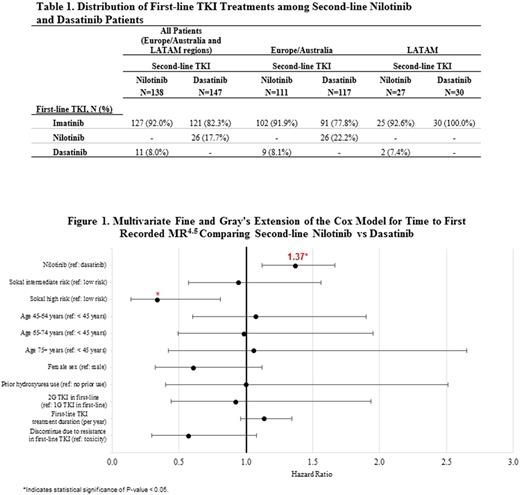
Online physician panel approach was used to recruit oncologists (N=172) globally to conduct physician-based chart reviews. To increase the representativeness of physician sample, each physician was allowed to review up to four patients, and was instructed to select these pts via randomization scheme. Eligible pts were diagnosed with CP-CML at age ≥18 years, initiated second-line nilotinib or dasatinib between 1/1/11 and 7/1/15, and had ≥12 months of follow-up since initiating the first-line or second-line TKI. A multivariate Fine and Gray's extension of Cox proportional hazards model was used to assess time to first recorded MR4.5 for nilotinib vs dasatinib as second-line therapy. The model accounted for competing-risk event of TKI resistance and random effect of country clustering of data, and was adjusted for age, gender, Sokal risk score at CML diagnosis, prior hydroxyurea use, first-line TKI agent (1st vs 2nd generation TKI), first-line TKI treatment duration, and reasons for first-line TKI discontinuation. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were reported.
Results
The study included 285 pts treated with nilotinib (N=138[48%]) or dasatinib (N=147[52%]) as second-line TKI, including 111 nilotinib and 117 dasatinib pts from Europe/Australia (80%), and 27 nilotinib and 30 dasatinib pts from LATAM (20%). Median follow-up time was 19.5 and 18.9 months for nilotinib and dasatinib in both regions, respectively. In Europe/Australia, 85% of pts had received imatinib as first-line TKI, and in LATAM, 96% (Table 1). In Europe/Australia, 85% of pts discontinued first-line TKI due to ineffectiveness (per treating physician) and 16% due to toxicity; corresponding percentages were 79% and 23% in LATAM. Median (range) age at second-line TKI initiation was 58 (18-88) years in Europe/Australia vs 49 (22-77) in LATAM. In Europe/Australia, more second-line nilotinib (21%) than dasatinib (10%) pts had high-risk Sokal score at CML diagnosis. Compared to Europe/Australia, more pts in LATAM had a high-risk Sokal score in both the nilotinib (30%) and dasatinib (27%) cohorts.
The raw proportion of pts achieving first recorded MR4.5 by 24 months of second-line TKI therapy was 34% for nilotinib and 31% for dasatinib. After controlling for country clustering effects and baseline covariates, adjusted multivariate Cox model showed that nilotinib was associated with a significantly higher rate of achieving MR4.5 (HR=1.37, 95% CI [1.12, 1.67], p<0.01) than dasatinib. Additionally, high-risk Sokal score was significantly inversely associated with achieving MR4.5 (HR=0.34, 95% CI [0.14, 0.81], p=0.01) while discontinuing first-line TKI due to resistance (HR=0.57, 95% CI [0.30, 1.08], p=0.08) reached borderline statistical significance at the two-sided α-level of <0.10 (Figure 1). MR4.5 outcome will be reported by regions in the presentation.
Conclusion
This retrospective chart review study shows some variations in pt and treatment characteristics across regions. A significant percentage of second-line TKI pts can reach MR4.5 after failure to first-line TKI. In addition, this study suggests that second-line nilotinib may be associated with a higher rate of MR4.5 than dasatinib in this setting. Given its retrospective and observational nature, our results should be taken with caution as this study is susceptible to unmeasured confounding and biases. Rigorous clinical assessment in a prospective setting is needed to conclusively compare rates of achieving MR4.5 in ptstreated with nilotinib versus dasatinib.
Cortes: ARIAD: Consultancy, Research Funding; Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Sun Pharma: Research Funding; BMS: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Huynh: Novartis Pharmaceuticals Corporation: Research Funding. Mendelson: Novartis Pharmaceuticals Corporation: Employment. Brandt: Novartis Pharmaceuticals Corporation: Employment. Dalal: Novartis Pharmaceuticals Corporation: Employment. Dersarkissian: Novartis Pharmaceuticals Corporation: Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Cortina: Novartis Pharmaceuticals Corporation: Employment. Narkhede: Novartis Pharmaceuticals Corporation: Research Funding. Duh: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; Novartis Pharmaceuticals Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal